Chemistry is the study of the composition and structure of matter. Chemistry studies the changes of matter- one can use the scientific method to study these changes in matter.
Energy changes are NOT changes in matter.
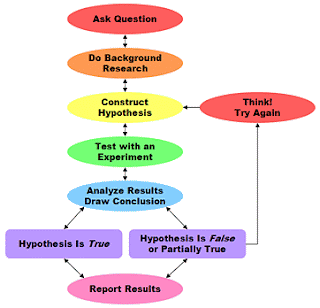
After observing a series of experiments a chemist can develop a THEORY to explain these observations.
Matter has two general types of properties:
1. extensive- "how much" such as mass and volume
2. intensive- does not depend on how much such as density.
Physical properties include- melting point, hardness, color, evaporation .
Chemical properties depend on how the substance combines with other substances: rusting, combining with oxygen.
States of Matter
Solid- definite shape and volume
Liquid- definite volume, takes the shape of its container/ indefinite shape.
Gas- indefinite volume, fills its container, easy to compress, expands when heated.
Vapor- gas phase of a substance that typically a solid or gas at room temperature.
Mixture- a blend of substances which are "easily separated".
Homogeneous mixture- same throughout, not chemically combined, "stainless steel".
Heterogeneous mixture- not the same throughout, soil, Italian salad dressing.
Chemical compound- substances that are chemically combined, can only be separated by chemical means.
During a chemical reaction matter is NOT created or destroyed, only changed.